What are surfactants? Surfactants are compounds that have two different ends – one hydrophobic and the other hydrophilic. The term hydrophobic is derived from the Greek, meaning “water-fearing.” In other words, oil and water do not mix. As a result, surfactants help water to wet the surface. But how do they do it? How do they work in products?
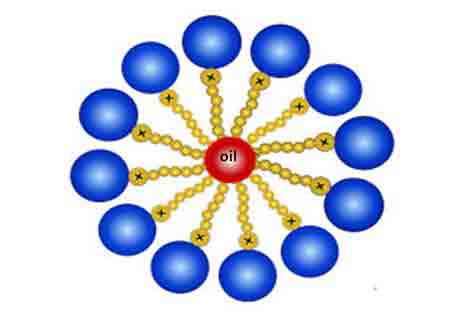
Micelle
The critical micelle concentration (CMC) of a surfactant is one of the most important chemical physical parameters. Different methods can be used to calculate the CMC values from the variation of a surfactant’s physical property as a function of concentration. Various mathematical approaches have also been developed to calculate the CMC value. While most are independent of the operator, not all are applicable to all methods.
A micelle is a tiny ball-like particle that forms in water when a IRO surfactantis added. Micelles have a head and tail, and trap oil inside them. Micelles are the basic constituents of water-based lotions and emulsions. Surfactants are classified into four primary groups, which include anionic, cationic, non-ionic, and zwitterionic. The different types of surfactants also represent a system between ordered and disordered states of matter. A surfactant solution may contain both an ordered and disordered phase, and therefore be categorized into one of the four main categories.
Surface tension
To investigate surface tension of surfactants, the researchers used a Kruss K100 tensiometer, a ring-based instrument based on the du Nouy-32 method. The tensiometer was equipped with two microdispensers for automatic dilution. The salt solution used in this experiment was the same concentration as the surfactant. The results obtained from this method were consistent with previous studies.
It is important to note that the true surface tension of a solution should not depend on the extensive parameters of a container. It is worth noting that lowering the level of the solution will greatly reduce the surface tension of a surfactant solution. Observations on changes in surface tension can be used to infer adsorption at the solid-solution interface. This method is also useful for evaluating the efficacy of various surface-active agents.
Applications
Surfactants are a class of chemicals with numerous uses. They are polymeric, and their comparative molecular mass is typically above 10000. Some surfactants are cationic, and are particularly useful against both gram-positive and gram-negative bacteria. They are also emulsifiers, suspension aids, and foaming agents. Despite the wide range of uses, surfactants are often categorized as compositing or natural materials. In the pharmaceutical industry, they may be used to kill germs and spores. Environmental disinfectants and preoperative skin and mucosa disinfection.
Surfactants are surface-active compounds with polar functions. They can act as detergents, wetting agents, or dispersants. They also serve as anti-foams and anti-dust agents. Some are used in personal care products, while others are used in firefighting, textiles, and food. Surfactants are also derived from other natural chemicals, including alkali-surfactant polymers, which are used in oil wells.